Timing is everything: survival of Atlantic salmon (Salmo salar) postsmolts during events of high salmon lice densities
Data files
Mar 18, 2020 version files 69.46 KB
Abstract
- Atlantic salmon in aquaculture act as reservoir hosts and vectors of parasites like salmon lice and this parasite is shown to harm wild salmonid populations.
- In the present study, n=29817 tagged Atlantic salmon were studied in four release trials. Half of the released fish were given prophylactic treatment against lice, the other half represented sham control fish. We used a nested design comparing years with low and high lice density and seasonal dynamics in infestation pressure. The released Atlantic salmon thus experienced highly variable lice infestation pressures, which we linked to survival and growth in returning fish. The fish were released in a protected “National Salmon Fjord“ and n=559 Atlantic salmon were recaptured after spending 1-4 years at sea.
- In most experimental groups 1 – 2.5 % of the fish were recaptured at return. However, survival of unprotected fish was extremely low for the trial released at the highest density of lice: only 0.03 % of these Atlantic salmon returned to the river, compared to 1.86 % in the protected group.
- Synthesis and applications . We document that a high lice density can cause a more than 50 times higher mortality risk in Atlantic salmon on their sea migration, even in a fjord with protected status. Fine-tuned and hard-to-predict year-to-year differences in timing, both for the wild smolt migration and the population build-up of lice released from aquaculture, means life or death to wild salmon. Management actions such as spatial segregation of farmed fish and lice (e.g. closed farm pens), and/or moving farms away from vulnerable habitats for wild salmonids (fjords and coastal areas), may be needed to ensure sustainable co-existence of wild and farmed Atlantic salmon.
Materials and Methods
Study area
The study was carried out in River Etne, draining in the outer parts of Hardangerfjord, in Hordaland county, western Norway (Fig. 1). The Hardangerfjord is among the most intensively used areas on the Norwegian coast for salmon production, with a standing stock of farmed Atlantic salmon of about 80 000 and 95 000 metric tonnes in 2013 and 2014, respectively (Fiskeridirektoratet 2019). For further details on the study area see (Halttunen et al. 2018).
Experimental design
The experiment started in 2013 and was replicated in 2014; two groups of Atlantic salmon were released in May and June, each year (Table 1). All fish were released close to the mouth of River Etne. Returning adult individuals were caught in the trap in River Etne after 1-4 years at sea.
Table1. Summary of released salmon smolts and sample sizes for treatment (prophylaxis) and control groups in the four trials. Fish weights in gram ± SD.
|
Year |
Release date |
Prophylaxis |
Control |
Weight (g) |
|
|
|
|
|
|
|
2013 |
May 18th |
3791 |
3972 |
72 ± 21 |
|
2013 |
June 9th |
3801 |
3868 |
74 ± 16 |
|
2014 |
May 18th |
3819 |
3818 |
47 ± 11 |
|
2014 |
June 9th |
3770 |
2978 |
42 ± 10 |
Fish used in this study were 1. generation one-year old hatchery-reared Atlantic salmon post smolts produced from eggs and sperm stripped from broodstock caught in River Etne. Fish were reared at Matre Research Station (IMR) and made ready for release in saltwater. Prior to release, all salmon smolts were tagged using coded wire tags (CWTs) inserted in their snout, which enable fish identification to i) treatment/control, and ii) timing of release. In addition, all fish had their adipose fin removed to enable us to distinguish experimental fish from wild fish in the trap on return to the river.
For the prophylactic antiparasitic treatment, we used a 30-minute bath of Substance EX (Pharmaq), hereafter termed SubEX, at a concentration of 2 ppm in oxygenated water. This treatment was applied to 50 % of the fish, randomly selected, securing a balanced design. SubEX protects the fish by preventing attached copepodids to develop into the next life stage for up to 16 weeks after treatment (Skilbrei et al. 2015). Identical (sham) treatment was performed on the control fish. This process was performed three days before each of the four releases to allow recovery of the treated fish.
After tagging and treatment, fish were transported in closed oxygenated tanks to Etne by car to a 5 m3 cage in the sea, close to the outlet of River Etne. The fish were kept in the cage for approximately 48 hours before they were released by lowering the net in the cage. The release was done by night to prevent immediate predation form birds. Prior to release a sample of 30 fish (randomly picked from the net) were killed to measure length and weight.
From 2014-2017, i.e. 1-4 years after release, all experimental fish returning to River Etne were caught in the fish trap and killed (wild Atlantic salmon not belonging to the experiment were released above the trap). Data on body length, weight and sex were registered at the return date.
Estimation of lice infestation pressure
Salmon lice densities were estimated based on sentinel cages (Bjørn et al. 2011) stocked with 30 farmed Atlantic salmon post smolts and positioned in the area the fish would migrate through (Fig. 1). We extracted lice counts from periods that approximately matched the times of release for the fish, i.e. in a 14-day period after May 18th and June 9th in 2013 and 2014. We included all life-stages of lice (from copepodites to adult stages) and calculated the total added number of lice per fish for a standardized period of 14 days (using modeled means of each cage mean, c.f. Fig. 2). These numbers were used to represent the environmental infestation pressure of lice in this study, hereafter termed Lice Infestation Pressure, for each of the four experimental releases. The positioning of the cages was the same between years.
To visualize the spatial distribution of lice infestation pressure in the whole area of interest (Fig. 3) we used the Relative Operating Characteristic (ROC) method to identify where the lice densities from the hydrodynamic lice dispersion model (see www.lakselus.no) were low (< 1 lice per fish), medium (1-10 lice per fish) or high ( > 10 lice per fish) (Sandvik et al. 2016). The hydrodynamic lice model is described in detail in earlier studies (Johnsen et al. 2014, Myksvoll et al. 2018).
Risk Ratio (RR)
The Risk Ratio or relative risk quantifies how much more likely the treated group is to return to the home river, compared to the control group. We analyzed differences in return rates between treated and non-treated fish, for each of the 4 experimental releases, with the following formulae:
RR=ET/(ET+NT)EC/(EC+NC)=ET(EC+NC)EC(ET+NT) (1)
where ET is the number of return events (E) in the treatment (T) group; NT is the number of non-return events (N) in the treatment (T) group; EC is the number of return events (E) in the control (C) group; and NC is the number of non-return events (N) in the control (C) group.
RR-values higher than 1 show higher adult salmon returns of treated fish as compared to control fish, RR-values lower than 1 show higher returns of the controls. We calculated confidence intervals for the RR with the formulae:

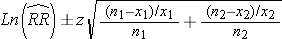 (2)
(2)
Where n1 and n2 = sample size of treated and non-treated fish released, respectively; x1 and x2 are sample size of returned fish in treated and control group, respectively. For 95 % confidence intervals we used z = 1.96.
Survival probability
The survival probability (probability of return) was modeled by logistic regression:
glm (Returned fish ~ Lice Infestation Pressure * Treatment, family = ’binomial’), (3)
where Returned fish represents the probability for surviving 1-4 years in the sea and returning to the river (1 for returning fish, 0 for non-returning fish), Lice Infestation Pressure is the estimated environmental infestation pressure (standardized with mean = 0 and SD = 2) of lice and Treatment is prophylaxis against lice versus control. We also tested whether Releaseweight (average fish weight for the group at release) was a significant covariate in the model. As Releaseweight was a non-significant covariate (Estimate = -0.0054, Z = -1.471, p = 0.14), and did not improve the model (AIC), we used the simpler model without this factor. For model validation, we inspected residuals and re-run the model excluding one outlier fish. However, as the results were practically the same, we decided to include all data points.
Growth at sea
The growth of the fish during its sea migration was evaluated with a linear regression model:
lm (Weight ~ Lice Infestation Pressure + Treatment + Seawinter + Sex) (4)
where Weight is individual fish body mass at return, Lice Infestation Pressure is the environmental lice infestation pressure (standardized with mean = 0 and SD = 2), Treatment is prophylaxis or control, Seawinter is the number of years at sea before returning to the river (standardized for 2SW fish by subtracting 2 from the number of seawinters) , and Sex differentiate males from females. Fish that spent 4 winters at sea was excluded from the analysis since these were only observed in one of the trials. We standardized Lice Infestation Pressure and Seawinter in order to have comparable effect sizes between factors and covariates in the model (Schielzeth 2010). For model validation, residuals were inspected visually (versus fitted values and leverage, qq-plot, scale-location). We also re-run the model without two potential outliers, but decided to include all fish in the data set.
Statistical analyses were carried out in R statistical package version 3.5.1 (R-Developmental-Core-Team 2019).
- Bøhn, Thomas; Gjelland, Karl Øystein; Serra‐Llinares, Rosa M. et al. (2020). Timing is everything: Survival of Atlantic salmon Salmo salar postsmolts during events of high salmon lice densities. Journal of Applied Ecology. https://doi.org/10.1111/1365-2664.13612
